Everlywell modified into as soon as one in every of the significant startups to reveal that it modified into as soon as engaged on a self-administered, at-dwelling COVID-19 diagnostic equipment, however it in reality first and predominant sought out to ship kits earlier than regulators made positive that this modified into as soon as no longer based entirely on its tricks. Everlywell then grew to was intent on working with the FDA to get an ethical Emergency Exercise Authorization for its kits earlier than sending any to shoppers, and that technique has paid off with the U.S. drug regulator issuing an EUA for Everlywell’s tech this day.
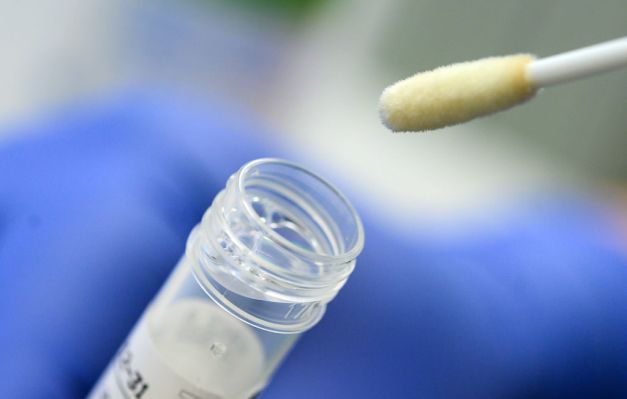
Everywell‘s COVID-19 Check Home Collection Equipment is the significant standalone pattern series equipment to be granted an ethical EUA by the FDA. Diversified kits had been in expend by strategy of physician-prescribed and directed series, and others easy had been licensed particularly to be used with one take a look at (where supplier of both equipment and take a look at are the same). This approval is outlandish because Everlywell is providing its pattern equipment self reliant of any particular making an are attempting out lab, and can work with a diversity of labs to doubtlessly present a broader making an are attempting out footprint.
The take a look at kits are then sent to one in every of two labs currently licensed below separate EUAs for COVID-19 making an are attempting out, and the administration notes that this would maybe maybe fabricate bigger to varied take a look at services in future could maybe easy they file for an EUA and present the requisite info that goes alongside with the verification required for that emergency approval. The FDA cites Everlywell’s work in gathering and presenting info from analysis including these supported by the Invoice and Melinda Gates Foundation to present that samples aloof at dwelling the utilization of its nasal swab series manner stay accurate at some stage in transport.
That info is furthermore now accessible to others making an are attempting to present the same take a look at equipment offerings, the FDA notes, which could maybe easy lower the burden of proof on someone making an are attempting to make authorization for a competing product. That can maybe maybe doubtlessly originate up making an are attempting out even extra, lowering a bottleneck that many public health professionals scrutinize as one in every of the significant drivers of a a success recovery.
“The authorization of a COVID-19 at-dwelling series equipment which shall be old with a pair of tests at a pair of labs no longer easiest gives increased affected person salvage entry to to tests, however furthermore protects others from doubtless exposure,” acknowledged Jeffrey Shuren, M.D., J.D., director of the FDA’s Middle for Devices and Radiological Properly being in a assertion equipped to TechCrunch . “On the present time’s movement is furthermore one other nice example of public-non-public partnerships in which info from a privately funded review modified into as soon as old by industry to enhance an EUA query, saving treasured time as we continue our wrestle by distinction pandemic.”


